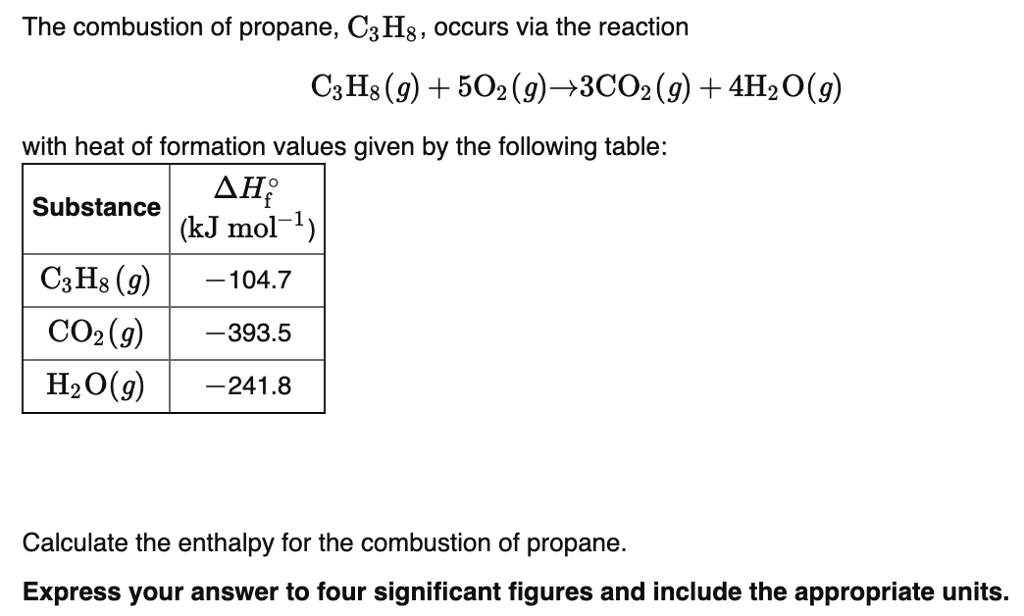
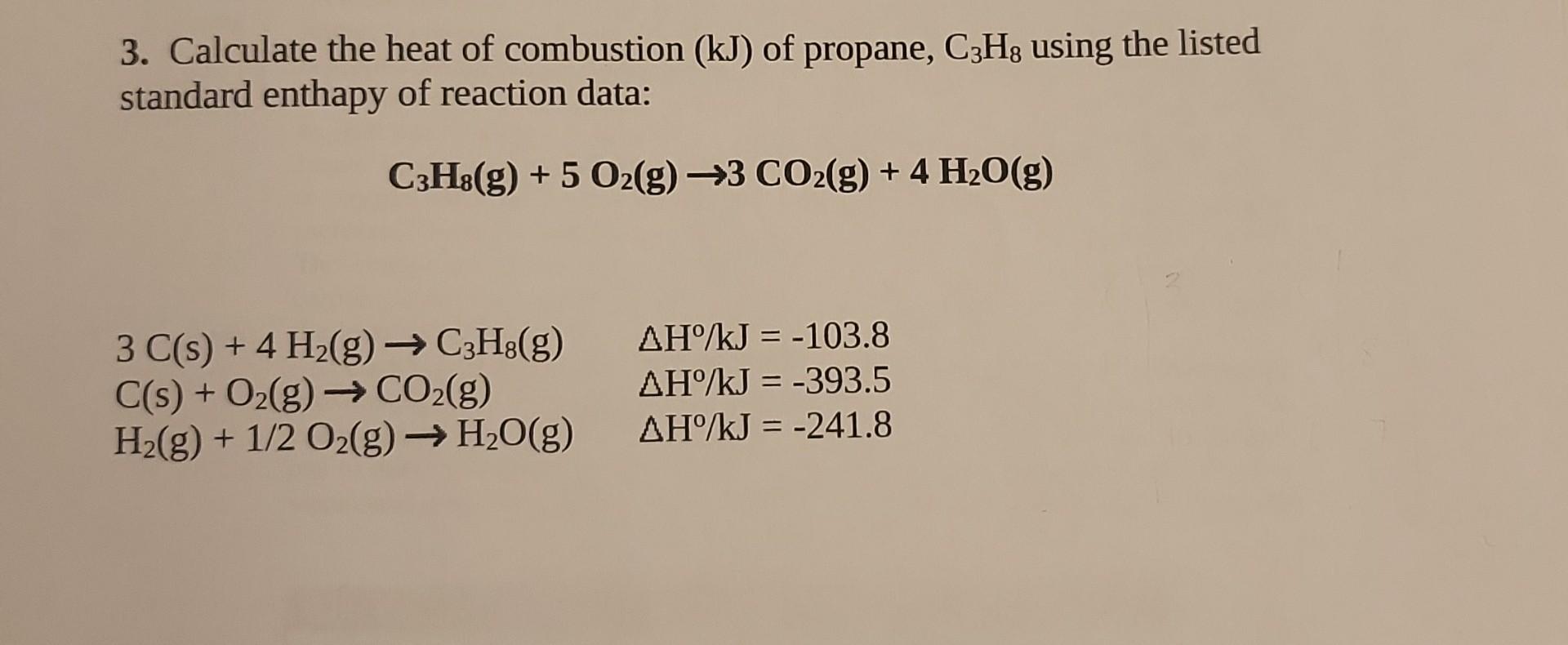
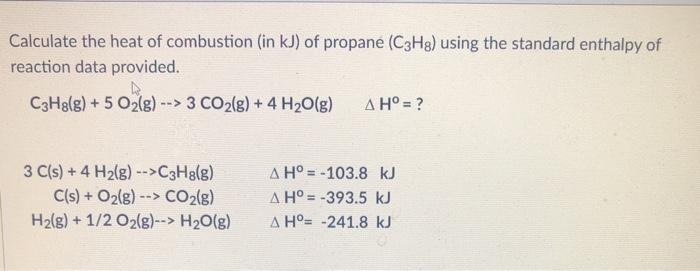
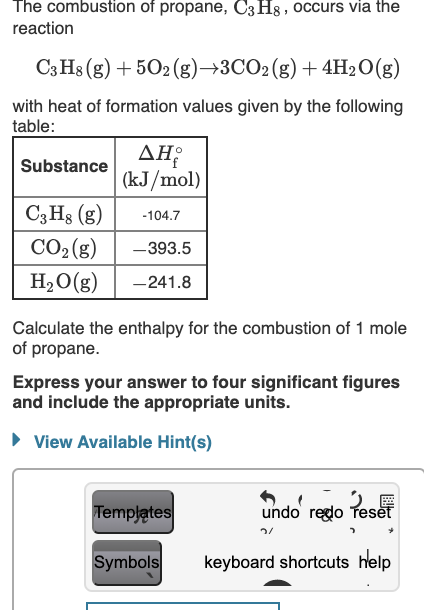
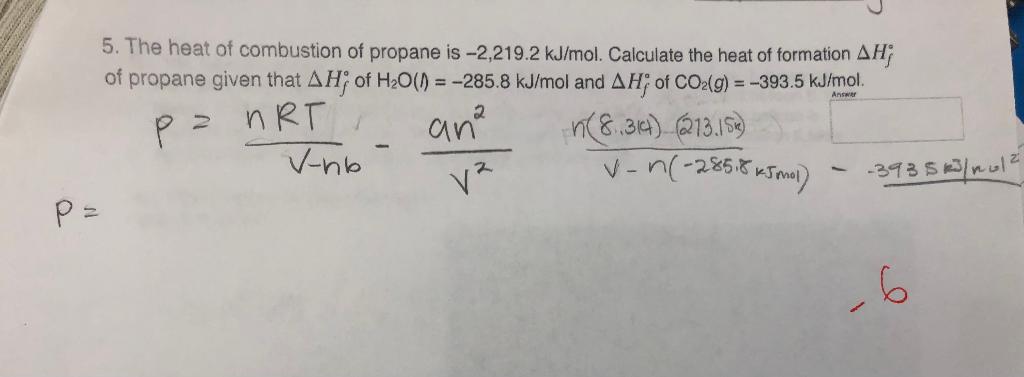60. If heat of formation of mathrm{C}_{3} mathrm{H}_{8}, mathrm{CO}_{2} and mathrm{H}_{2} mathrm{O} are -38.2,-94.1 and -48.2 kcal respectively. Then calculate heat of combustion of propane: (1) -423.3 mathrm{kcal} (2) +423.3 mathrm{kcal} (3) -
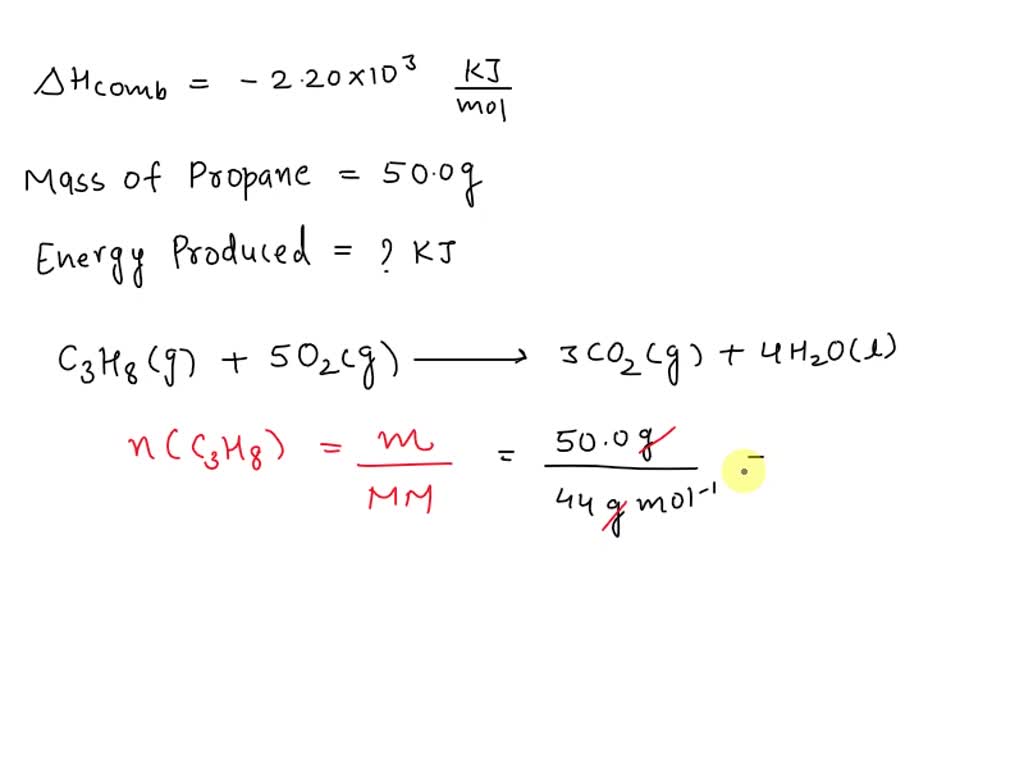
SOLVED: The heat of combustion of propane is -2.20E3 kJ/mol. Calculate the energy in kJ produced when 50.0 g of propane is burned in excess oxygen.

The standard heat of combustion of propane is -2220.1 kJ / mol. The standard heat of vaporizatio... - YouTube

A 10.0 g sample of propane, C3H8, was combusted in a constant-volume bomb calorimeter. The total heat - Brainly.in

The heat of combustion of propane C3H2(g) is AH = -2220 kJ /mol. For the combustion of 1.00 m3 of C3H8(g) measured 25° C and 1 atm pressure, AH = (A) -2220
enthalpy of combusstion of propane, bu†an e, and pen†an e are 2220, 2878, 3537 kj/mole respectively . order of calorific value (per gram ) of fuel will b

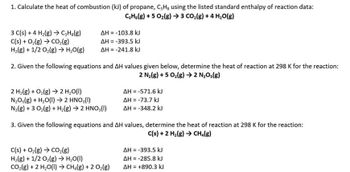
3. The standard heat of combustion of propane is -2220.1 kJ mol-! The standard heat of varonisation of liquid water is 44.0 kJ mol-. What is AH of - CoHs (9) +502 (
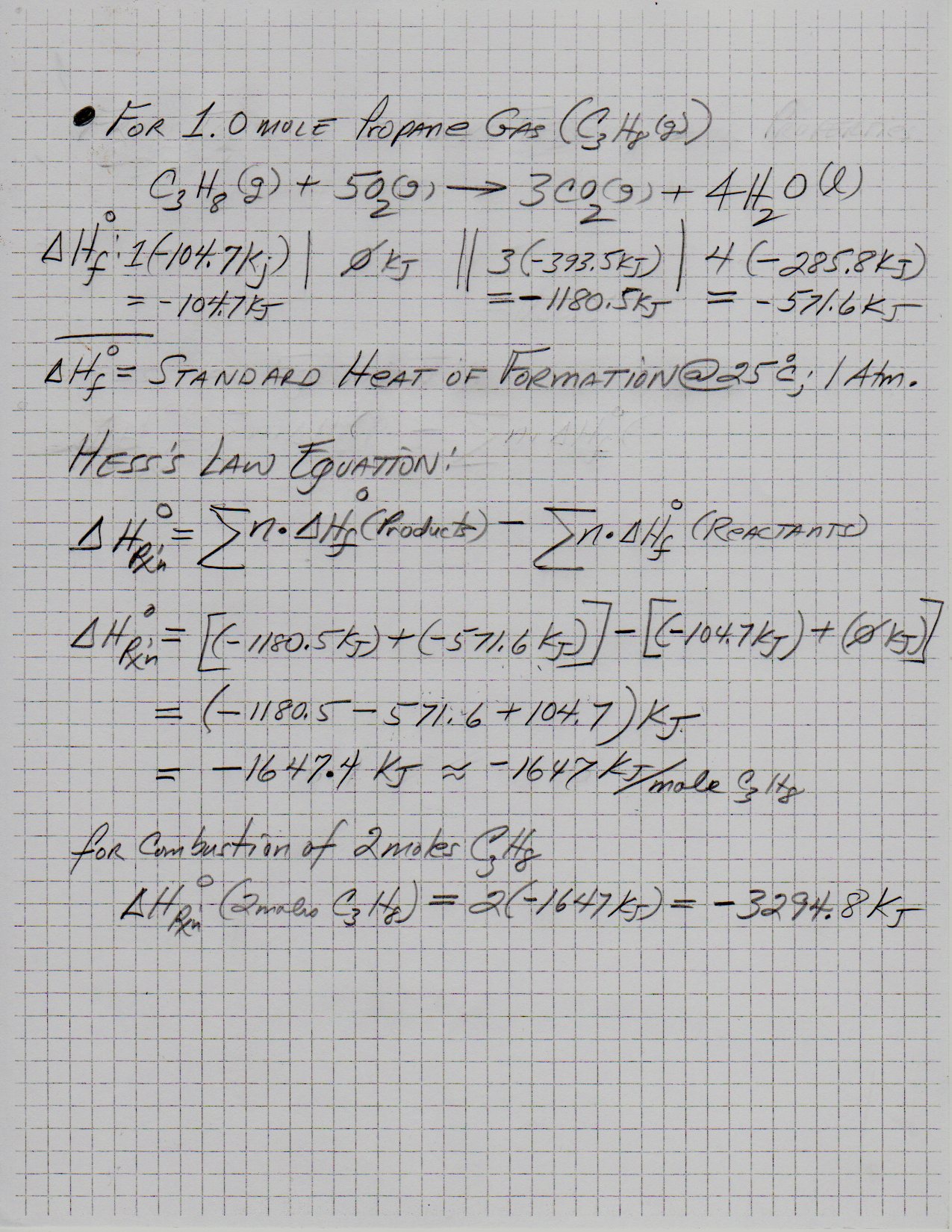
Using the chemical equation below, determine the energy released by burning 2 moles of propane, "C"_3"H"_8? a) 4438.4 kJ b) 11096 kJ c) 2219.2 kJ d) 1109.6 kJ | Socratic
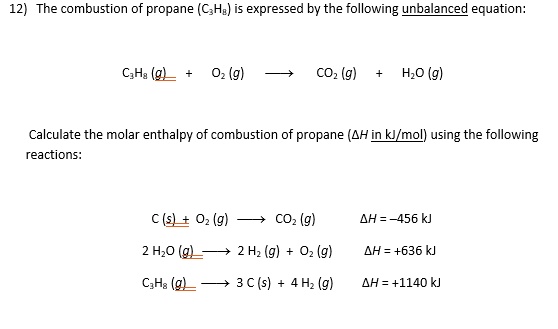
SOLVED: The combustion of propane (C3H8) is expressed by the following unbalanced equation: C3H8 (g) + O2 (g) -> CO2 (g) + H2O (g) Calculate the molar enthalpy of combustion of propane (
Calculate the standard heat of formation of propane, if its heat of combustion is 0-2220.2 KJ mol^-1 the heats of formation - Sarthaks eConnect | Largest Online Education Community

SOLVED: Consider the combustion of propane: C3H8(g) + 5O2(g) â†' 3CO2(g) + 4H2O(g) ΔH = -2221 kJ Assume that all of the heat comes from the combustion of propane. Calculate ΔH when
![The HRR ramp of the propane burner in the experiments [11, 12] and the... | Download Scientific Diagram The HRR ramp of the propane burner in the experiments [11, 12] and the... | Download Scientific Diagram](https://www.researchgate.net/publication/329589405/figure/tbl1/AS:703057390231556@1544633352265/The-HRR-ramp-of-the-propane-burner-in-the-experiments-11-12-and-the-corresponding-mass.png)
The HRR ramp of the propane burner in the experiments [11, 12] and the... | Download Scientific Diagram