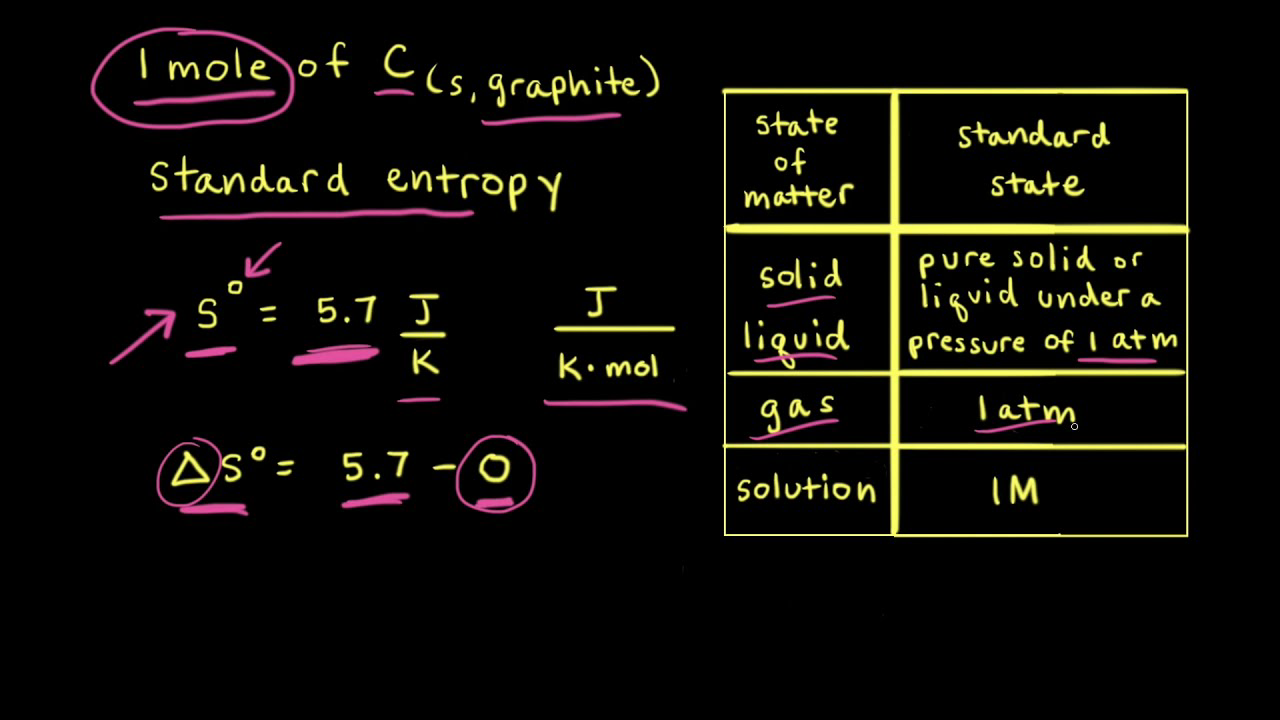
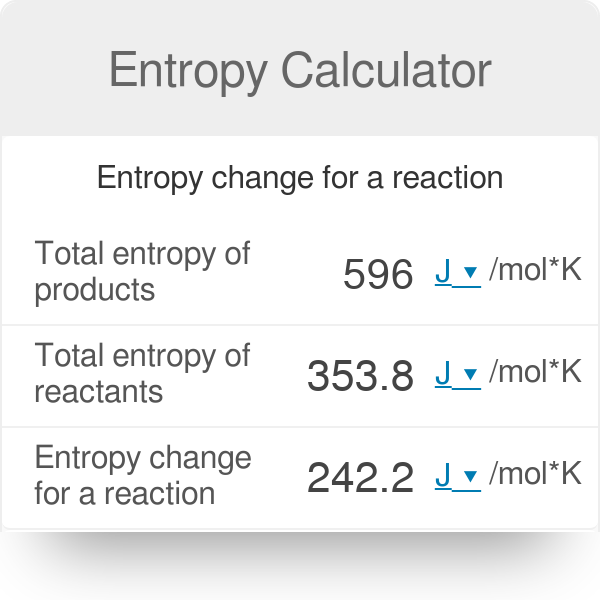
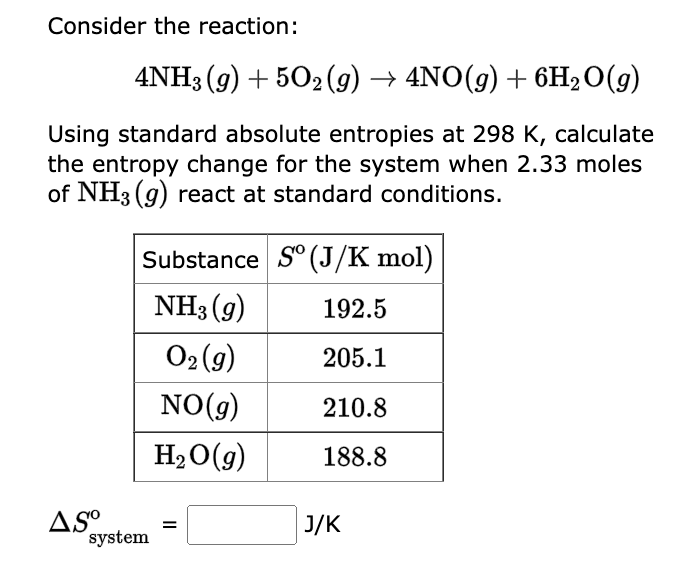
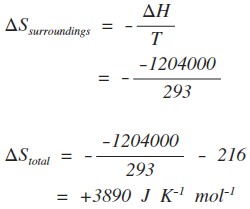
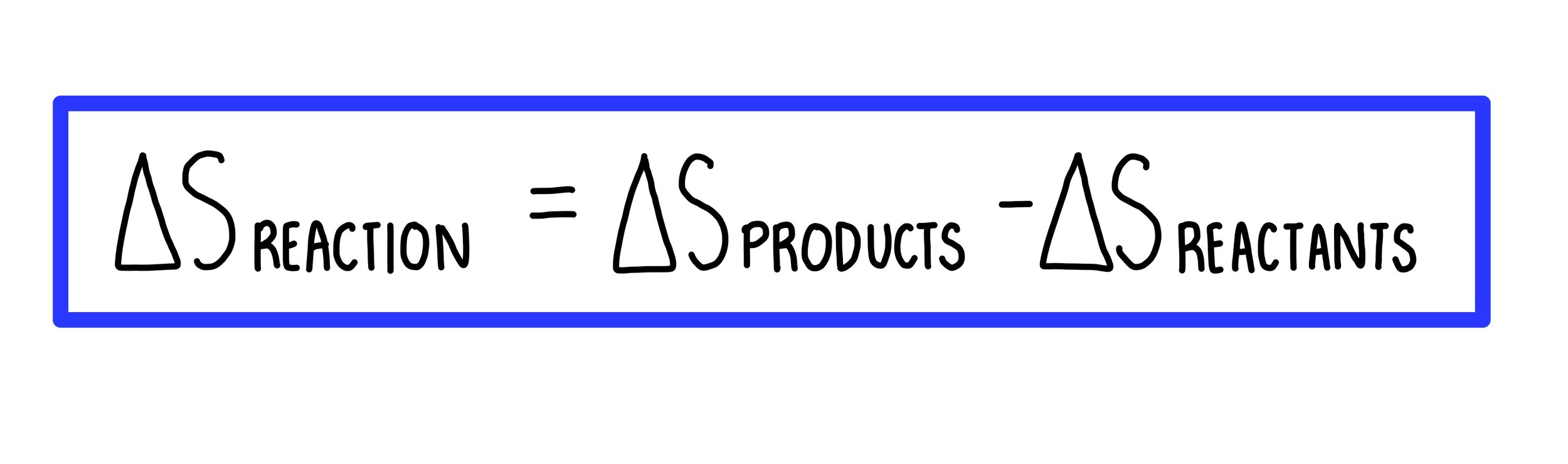
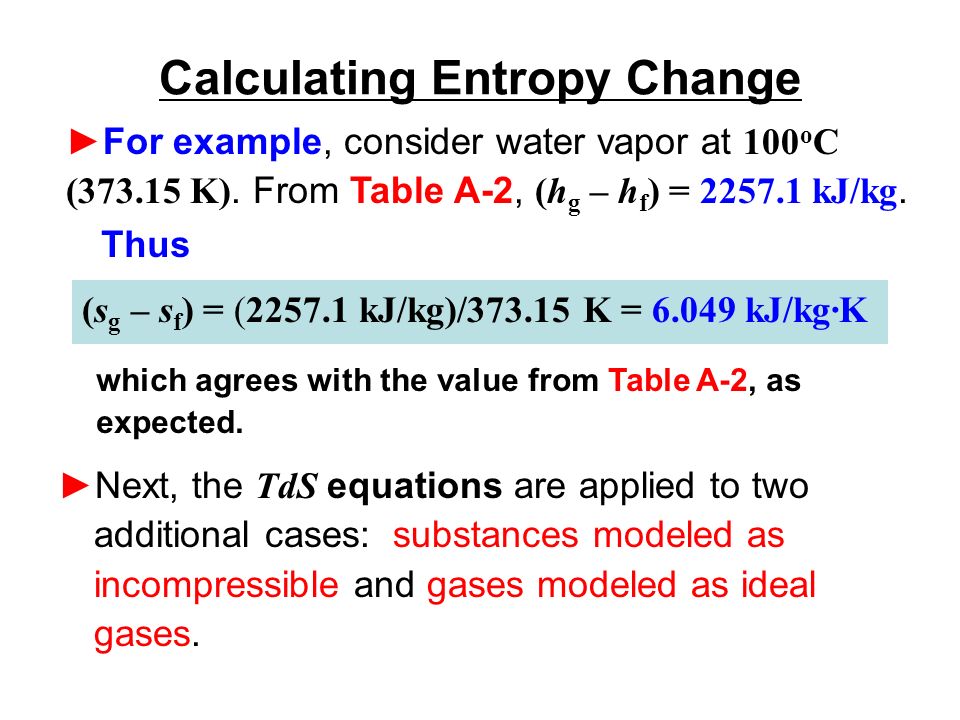
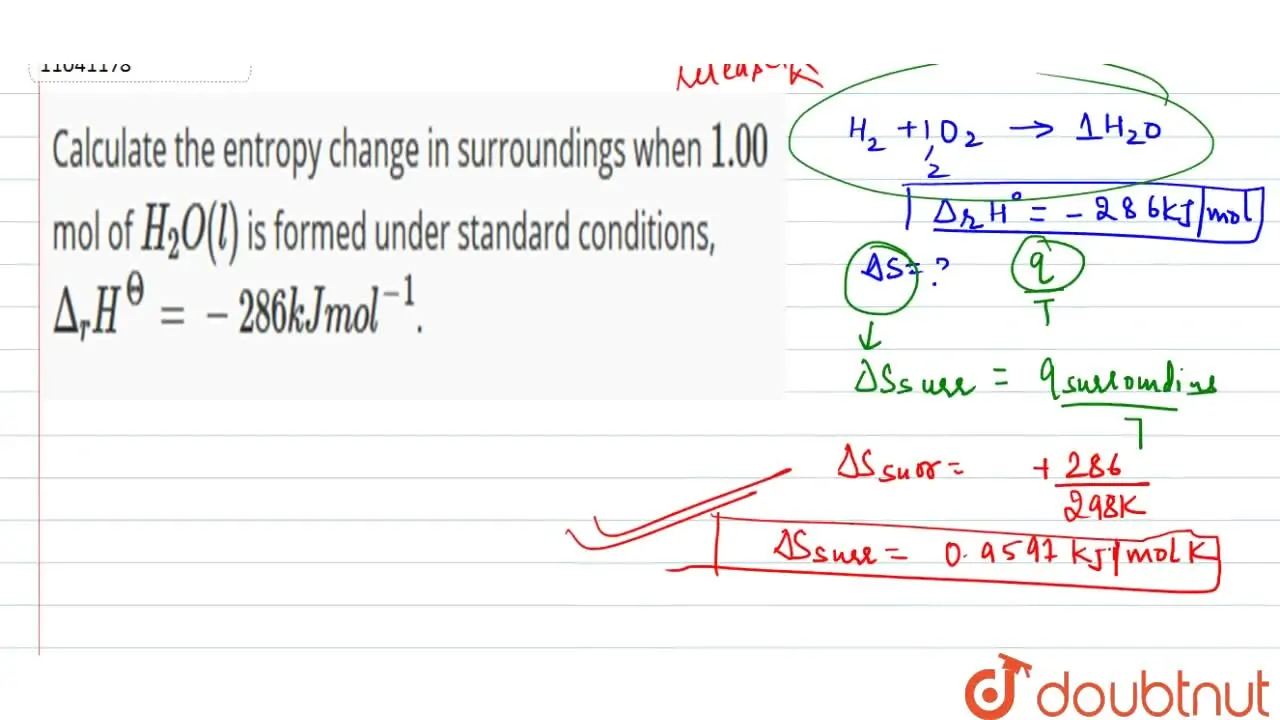
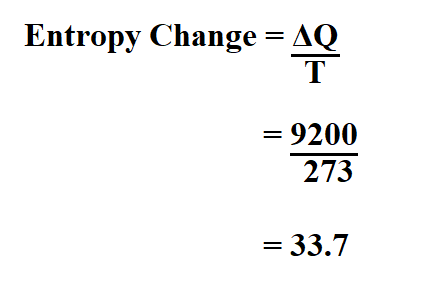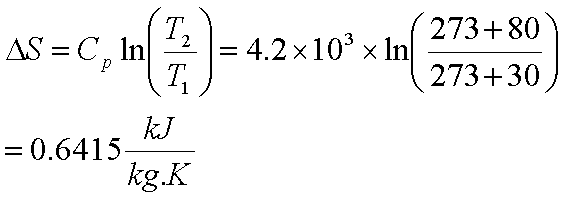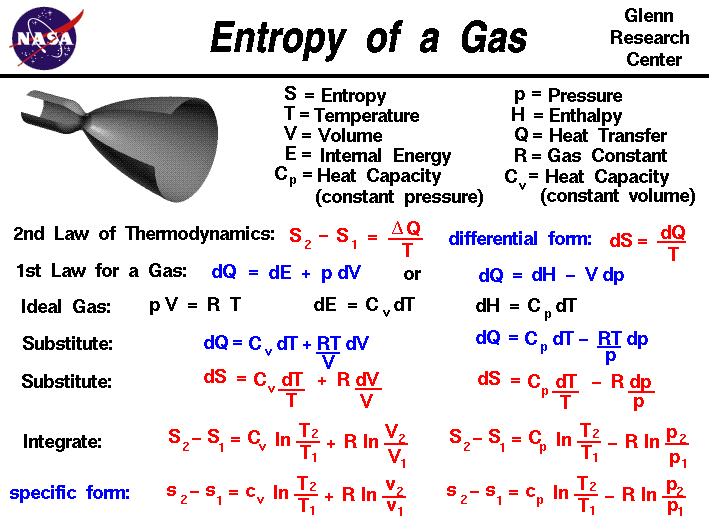22. Calculate the entropy change when 2.8 g of N, gas expands isothermal e entopy change when 2.8 g of N, gas expands isothermally and reversibly from an initial volume of 1

SOLVED: Calculate the entropy change of the universe (J/mol-K) when the entropy change of the system is 59.4 J/mol-K and the surroundings absorb 33.71 kJ of heat from the system at 77.74 °
The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is (Enthalpy of vaporization for water is 40.63 kJ mol–1)
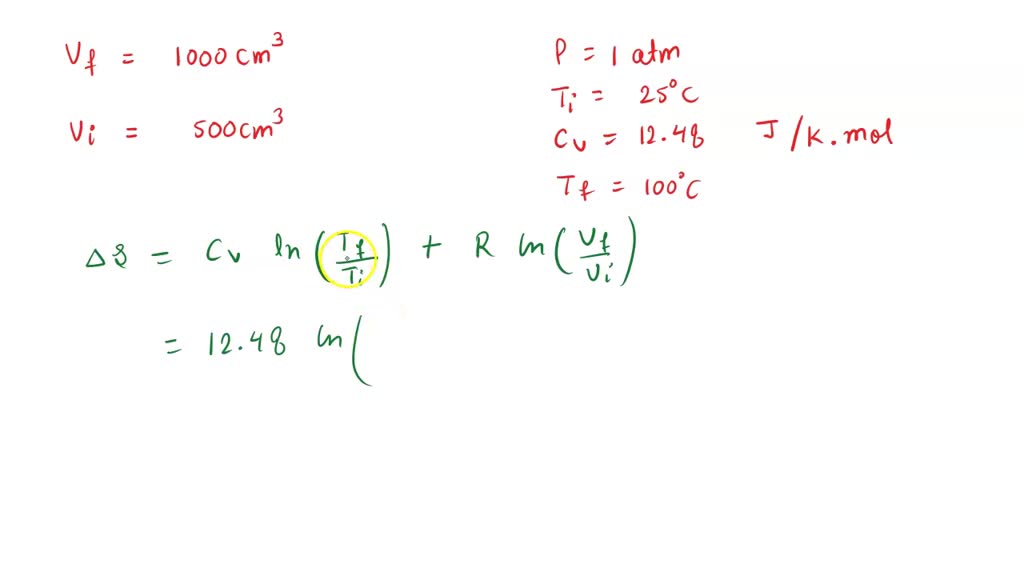
SOLVED: Calculate the entropy change when argon at 25 °C 1.00 atm in a container of volume 500 cm3 was allowed to expand to 1000 cm3 and is simultaneously heated to 100